Stopped-Flow Equipment for experiments in biophysics
Stopped flow expression is used both for the
technique (method) and for the instrument, too.
It is one of the most frequently used fast-kinetic techniques. With stopped
flow we are able to examine small volumes of solutions which are driven
from special syringes to a high efficiency mixer just before
passing into a measurement flow cell. As the solutions flow through,
a steady state equilibrium is getting established and the resultant solution
is only a few milliseconds old as it passes through the cell, where the
observation actually happens. The mixed solution then passes into a stopping
syringe where the flow of mixed solutions can be instantaneously
stopped. A fraction of the solution rests in the observation cell and
as the reaction of the two solutions proceeds, the kinetics can be followed
using the appropriate measurement technique.
What can we measure?
Change in (almost) any kind of photometric property
of the mixing solutions. Increase / decrease of absorption / emission
/ fluorescency could act as a measured 'sign'.
The most common method to follow the kinetics is by absorbance or fluorescence
spectrometry, and in these cases the measurement cell is an appropriate
spectrometer flow cell.
Why changes the 'sign'?
Chemical (physical) reaction of the solvents or the soluted materials
could change the properties. Formation or folding of chemical bonds, acquisition
or loose of charges, changes in 3D-structure ... indirectly can be monitored.
Only mixing of materials which interact and if the photometric property
of (at least) one of those changes, have sense! Nothing else.
Single Mixing Stopped-Flow
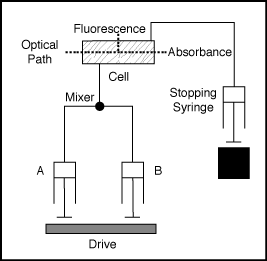
Two solution is mixed, so only two syringe is used. The solutions leave the syringes at the same time. It have to be considered that both of these is attenuated to the half of the original concentration after mixing.
One of the most useful advances of the stopped flow technique is that
the required volume is only a few cm3. It can be enough for
many measurements because the minimal volume which should sweep out the
previous sample used by the previous measurement is about 0,05-0,1 cm3.
It depends mainly on the volume of the observation cell (0,002-0,020 cm3).
Double Mixing Stopped-Flow
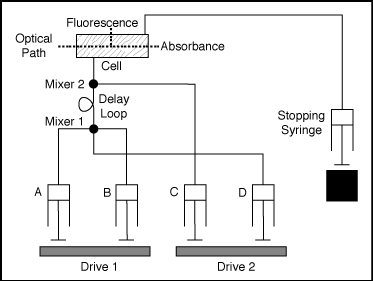 A wide variety of options
include multi-mixing where more than two solutions are mixed together.
In this case syringes marked as A and B are emptied at the same time.
Materials of syringes C and D can be added separatively from A+B. Even
though C+D are emptied at the same time, the timeline of the mixing can
be adjusted by using a delay loop .
A wide variety of options
include multi-mixing where more than two solutions are mixed together.
In this case syringes marked as A and B are emptied at the same time.
Materials of syringes C and D can be added separatively from A+B. Even
though C+D are emptied at the same time, the timeline of the mixing can
be adjusted by using a delay loop .
Variable ratio mixing is also available using syringes of different sizes and microvolume mixing for applications where only very small quantities of reactants are available.
Elements of the Stopped flow equipment
Accessories:
Desktop computer (controll over the process by a software, collection
and assimilation of data)
Thermostat (temperature stabilization)
Elements of the main part:
Lamp (light source)
Monochromator (wavelength selection)
Mechanical part (syringes, tubes, drives...)
Detector(s) (measures absorption or fluorescence)
 |
 |
 |
 |
Extras:
The environment of the reagents is also extremely important and so appropriate equipment is available for anaerobic applications, sub zero temperature and high pressure stopped flow applications. There are special stopped flow systems for measurements of circular dichroism and conductimetry.
Keywords:
stopped flow or stopped-flow, fast kinetic measurement, fluorescence,
absorbance, syringe, mixer, measurement flow cell, reaction, reactants,
solution
